Last week’s round -up;
30 August – 03 September 2021
New and Revised FDA Product-Specific Guidance
The US FDA has issued 23 new and 16 revised draft product-specific guidances which are intended to facilitate generic drug development, especially for medicinal products for which there are no approved generics. The newly issued guidance documents involve some products used to treat HIV, chronic obstructive pulmonary disease (COPD) and Cushing’s disease. Some of the new guidances for complex products cover ipratropium bromide nasal spray, ipratropium bromide inhalation spray and olodaterol hydrochloride inhalation spray. The FDA has also issued product-specific guidance for Type 2 diabetes drug semaglutide, which offers two paths to establishing bioequivalence.
https://lnkd.in/dU7Q26pn
Herbal medicinal products containing toxic, unsaturated pyrrolizidine alkaloids
The EMA has released a revised public statement on the use of herbal medicinal products containing toxic, unsaturated pyrrolizidine alkaloids (PAs). The document also includes recommendations regarding contamination of herbal medicinal products with PAs. Several PAs are regarded as both hepatotoxic and carcinogenic and are natural constituents of a number of plants used for medicinal purposes. This guidance describes chemical, toxicological, pharmacological and pharmacokinetic properties of PAs and sets out recommendations for the oral and cutaneous use of herbal medicinal products and traditional herbal medicinal products containing PAs. The revised public statement is a result of a review of newly available data and improved evaluation methods.
https://lnkd.in/egibCu6g
MHRA Guidance on Transfer of Analytical Methods
The MHRA Inspectorate has released new guidance for manufacturers and contract testing laboratories related to the process of transferring a method for outsourcing of testing. The Inspectorate has highlighted that the new guidance complements the requirements of EU GMP Chapter 7. The new guidance covers the formal process for the introduction of new methods which allows the receiving laboratory to demonstrate that they can perform the analytical method effectively and reproducibly. It also highlights that regulatory compliance is a shared responsibility between the transferring and receiving laboratories and a collaborative approach is encouraged. The MHRA has also recommended the use of risk management principles for analytical method transfer and the generation of a transfer report following a successful or even unsuccessful method transfer. A list of common shortcomings related to analytical method transfer is also included in the guidance.
https://lnkd.in/eQM2tWqp
(more…)
Last week’s round -up;
09-13 August 2021
Revised WHO guidance on GMP for investigational products and R&D facilities
The WHO released a revised draft guidance to industry addressing GMP for investigational drug products and new draft guidance on GMP principles for research and development (R&D) facilities in the context of the COVID-19 pandemic. The guidance on GMP for investigational drug products contains news recommendations on GMP issues related to quality management, quality risk management, personnel and documentation considerations. Issues related to manufacturing premises, equipment and utilities, materials and production are also addressed. Sections on the quality unit, qualifications and validation, complaints, recalls, returns, shipping and destruction are also covered. Some of the new recommendations include the requirement for a responsible person to be designated for the release of batches. The GMP guidance related to R&D facilities covers quality management and quality risk management in product research and development. It includes recommendations on sanitation and hygiene, qualification and validation, outsourced activities, self-inspection and quality audits, personnel training, premises, equipment and instruments and materials, documentation, processing and process design, quality control, stability and technology transfer.
Both draft guidelines are available for comment until 31st August 2021: https://bit.ly/RealCMC-2VU4jUD
New EMA Q&A on Clinical pharmacology and pharmacokinetics
The EMA has released a new Q&A 6.5 on Clinical pharmacology and pharmacokinetics. The new Q&A gives recommendations on how large the deviations from proportionality in composition can be in the case of fixed combinations with highly soluble active substances in an application with multiple strengths. For fixed combinations (FCs) consisting of multiple strengths, small differences in proportionality of compositions may preclude the waiver of the additional strength and result in the request of an additional in vivo bioequivalence study. However, in the case that all the active substances in the FC belong to BCS Class I or III drugs (highly soluble active substances), the risk of non-bioequivalent additional strength formulations is negligible, if the conditions specified in the Q&A are fulfilled. Therefore, a waiver for additional strengths is acceptable even though the additional strengths deviate from proportionality in composition, provided the conditions are met.
https://bit.ly/30oSmGp
UK MHRA publishes the new Access Consortium Strategic Plan for 2021-2024
The Access Consortium is committed to maximizing collaboration by aligning regulatory and policy approaches, reducing duplication, and facilitating the member country populations’ access to high quality, safe and effective health products. This group originally consisted of the regulatory authorities in Australia, Canada, Singapore and Switzerland (previously referred to as ACSS). With the addition of the UK MHRA in October 2020, ACSS changed its name to Access Consortium, and now represents a collective population base of 150 million people across the 5 member countries. The Access Consortium has now published a three-year strategy covering 2021-2024, and further information and the document itself can be found through the following link: https://bit.ly/RRL-3g88UJV
Reflection paper on GMP and Marketing Authorisation Holders
The EMA has adopted the Reflection paper on Good Manufacturing Practice and Marketing Authorisation Holders. Although many MAH companies are not directly involved in the manufacture of medicinal products themselves, the current European Commission Guide to GMP refers, in several places, to MAHs and their responsibilities in relation to GMP. The new Reflection Paper provides clarity as to what the various responsibilities are and what they mean for MAHs at a practical level. This reflection paper also addresses the various legislative provisions (i.e. in European Directives, Regulations and in other guidelines) which relate to GMP and which concern MAHs.
https://bit.ly/RealCMC-3xypFn5
UK MHRA issues updated guide to “defective medicinal products”
The MHRA Guide to Defective Medicinal Products regarding reporting, investigating and recalling suspected defective medicinal products to the Defective Medicines Report Centre (DMRC) has been updated, and can be found at the following link: https://bit.ly/RRL-2VHcTFT
CMDh Meeting Report – July 2021
The CMDh has released a report on the meeting that was held on 20th-21st July 2021. Various topics were tackled during the meeting including the following:
· An update to the CMDh Questions & Answers on implementation of outcome of Art. 31 referral on angiotensin-II-receptor antagonists (sartans) containing a tetrazole group – As outlined in Q7, under Condition B a response to the Article 5(3) referral is always needed for sartans containing a tetrazole group. Therefore, since it is considered that a risk of nitrosamines is always present for tetrazole sartans, due to their chemical structure, a Step 2 response is always expected and MAHs who previously submitted a Step 1 ‘no risk’ response, are expected to reconsider and submit a Step 2 response.
· Active Substance Master File (ASMF) worksharing – The CMDh strongly recommends the use of ASMF worksharing to save resources and promote a harmonised assessment when submitting an application for a medicinal product containing an ASMF.
https://bit.ly/RealCMC-3Ass5p8
(more…)
March 2021
Real Regulatory Tips and Insights
The tips we published in March 2021 are collected here, for convenience. Make sure you follow Real Regulatory Ltd and Real CMC for regulatory news, reports and hints.
Clinical Trials Comparators

IRIS
https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/iris-guide-registration_en.pdf

User Roles in IRIS
https://lnkd.in/dxRmsTG

Variation eApplication Forms

For previous monthly round-ups, you can follow these links:
February 2021 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/february-2021-real-regulatory-tips-and-insights/
January 2021 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/january-2021-real-regulatory-tips-and-insights/
December 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/december-2020-real-regulatory-tips-and-insights/
Last week’s round -up;
08-12 March 2021
EMA update on the new Clinical Trial Regulation (CTR) submission platform Go-Live
EMA has confirm that it plans to go-live with the new Clinical Trial Information System (CTIS) on 31st Jan 2022. However, the final go-live date will be six months after the European Commission confirms the full functioning of CTIS through an independent audit. CTIS will become the single entry point for submitting clinical trial information in the EU, which will be stored within the system.
Full details of the CTR and updates on CTIS training including updates on the CTIS training currently ongoing can be found here: https://lnkd.in/dm3Q7wE
Revision of CEPs referring to “sartan” monographs
Following the revision of the five “sartan” monographs (Valsartan, Losartan potassium, Candesartan cilexetil, Olmesartan medoxomil and Irbesartan) via a rapid implementation procedure, which enter into force on 1st April 2021, the currently valid CEPs referring to these monographs are considered to be already in conformity with the requirements of the monographs and remain valid. CEP holders of these substances will not be contacted by the Certification Department, as per usual procedure following the introduction of a revised monograph. CEP holders may decide to revise their control strategy for nitrosamine impurities on the basis of the production statements included in the monographs. In such cases a request for a minor revision should be made according to the EDQM Guideline on requirements for revision/renewal of Certificates of Suitability to the European Pharmacopoeia monographs (PA/PH/CEP (04) 2, 7R corr), 4.II.2.1 Change in the specification parameters and/or limits of the final substance g) Change of a limit for a mutagenic impurity in the final substance specification according to the principles and limits of the ICH M7 guideline. http://bit.ly/RealCMC-2N9JWOM.
UK MHRA publishes new requirements for Medical Device and IVD import to Northern Ireland
The MHRA has included a new section on importer requirements in their guidance on the new EU Regulations for medical devices (MDR) and in vitro diagnostic medical devices (IVDR), and their implementation in Northern Ireland. The guidance is available at the following link: https://lnkd.in/e-y2QKM The MHRA has also included a new section on customs requirements in their guidance for retailers on supplying medical devices from Great Britain into Northern Ireland. The guidance is available at the following link: https://lnkd.in/eW9b-3S
Nitrosamines update
The EMA has released the “European Medicines Regulatory Network approach for the implementation of the CHMP Opinion pursuant to Article 5(3) of Regulation (EC) No 726/2004 for nitrosamine impurities in human medicines”. The document lays out the conclusions of the CHMP review and the process for detection of N-nitrosamines in pharmaceuticals. The guidance also describes the elements to consider when deciding on the best regulatory pathway and the operational and organisational aspects at regulatory authorities’ level, which includes an overview of the activities carried out by the Nitrosamine Implementation Oversight Group (NIOG). https://bit.ly/RealCMC-3rtcBNB
(more…)
Last week’s round -up;
01-05 March 2021
Rapid implementation of revised sartan Ph. Eur. monographs
Five Ph. Eur. monographs on sartans with a tetrazole ring, namely Valsartan (2423), Losartan potassium (2232), Irbesartan (2465), Candesartan cilexetil (2573) and Olmesartan medoxomil (2600), have been aligned with the latest regulatory recommendations issued by the CHMP. The revision concerns a rewording of the “Production” section and deletion of the N-nitrosamines test section. Since the changes concerned are in line with the CHMP recommendations, the Ph. Eur. Commission has published the monographs under the rapid-revision procedure and they will be implemented on the 1st April 2021. The revised monographs can be downloaded from the EDQM website and have also been added to the online and downloadable versions of Supplements 10.4 and 10.5. The revised sartan monographs will be included in the printed version from Supplement 10.6. https://bit.ly/3uOvi0B
UK MHRA publishes major update to GMP requirements for “Specials” manufacturers
The MHRA has made a major update to the manufacture of sterile medicinal products section to add definitions of “aseptic manipulation” and “compounded intermediate for use in further process”, for example parenteral nutrition (PN) manufacture. Guidance has been added on additional risk reducing factors to be used when producing compounded intermediates, and on the elements to be included in a contamination control strategy. Other minor changes to the formatting and numbering of the existing guidance have been made.
The guidance is available at the following link: https://lnkd.in/ec5zapG
FDA Inactive Ingredient Database Update
FDA has improved the functionality of its Inactive Ingredient Database (IID) through the introduction of a quarterly log to track changes in the records and the use of the term “maximum daily exposure” (MDE) instead of “maximum potency.” The quarterly log allows users to track changes made to the IID, such as the MDE for potency replacement, on an on-going basis. The use of the MDE as opposed to “maximum potency” provides applicants with more complete information about the acceptable level of a particular excipient in a drug product. The maximum potency values are being replaced as the algorithmic calculation of MDEs is an on-going process. A webinar presented by Susan Zuk, the Branch Chief of the CDER Office of Pharmaceutical Quality (OPQ) Office of Policy for Pharmaceutical Quality (OPPQ), highlighted the recent progress made to the database and considerations that warrant further attention.
A free recording of the webinar is available and further details may be found at the following link: http://bit.ly/3suiv1g
(more…)
February 2021
Real Regulatory Tips and Insights
The tips we published in February 2021 are collected here, for convenience. Make sure you follow Real Regulatory Ltd and Real CMC for regulatory news, reports and hints.
Clinical Trials Facilitation Group (CTFG) Guidance for Clinical Trials

Orphan Condition & Orphan Indication in the European Union

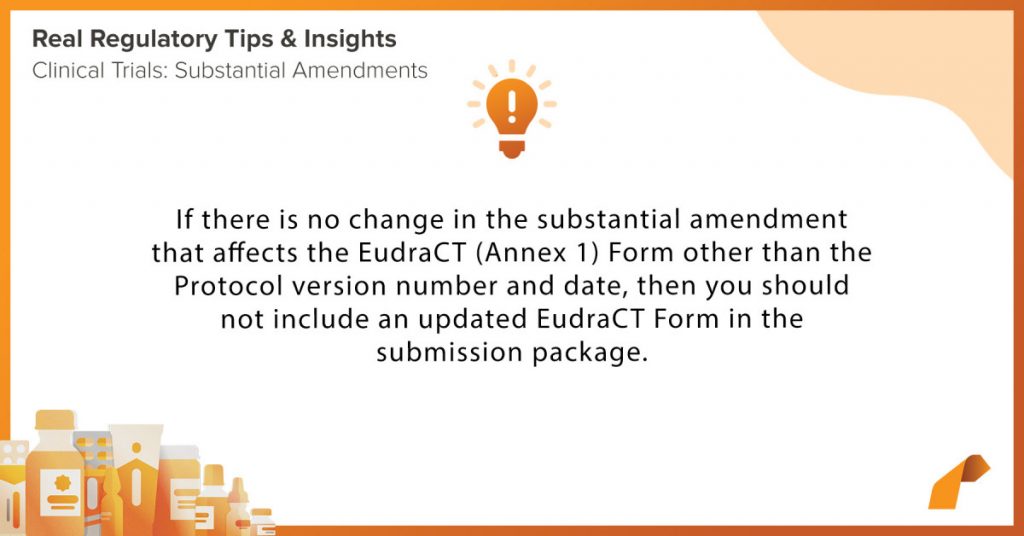
Clinical Trials: Substantial Amendments
Clinical Trials: Planning for EU Sites
https://www.hma.eu/ctfg.html

For previous monthly round-ups, you can follow these links:
January 2021 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/january-2021-real-regulatory-tips-and-insights/
December 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/december-2020-real-regulatory-tips-and-insights/
November 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/november-2020-real-regulatory-tips-and-insights/
Last week’s round -up;
15-19 February 2021
MHRA has updated their guidance on managing CTs during this pandemic
The guidance now includes a section on “Management of COVID-19 vaccine deployment for ongoing non-COVID-19 clinical trials”. The Sponsor should conduct a specific risk assessment for concomitant use of a COVID-19 vaccine for each IMP and with specific consideration for the trial population. Some trials may also need to consider risk assessment for non-IMPs and/or combinations.
Details in full can be found here: https://lnkd.in/ebfYpNS#IMP
CMDh revised MRP/DCP guidance
The CMDh has released the following revised MRP/DCP and national procedure guidance:
• Requirements on Submissions (number and format) for New Marketing Authorisation Applications within MRP, DCP and National Procedures (tracked).
• Requirements on submissions (number and format) for Variations and Renewals within MRP and National Procedures (tracked).
• CMDh annotated QRD template for MR/DC procedures (tracked).
https://bit.ly/RealCMC-3pEBUed
UK MHRA has updated the fees payable to the MHRA for 2020 to 2021
Update the MHRA fees for marketing authorisations and variations now include the new national procedures following exit from the EU, and the new UK “Orphan Marketing Products” fees, available at the following link: https://www.gov.uk/government/publications/mhra-fees?utm_medium=email&utm_campaign=govuk-notifications&utm_source=508f632b-1ef5-4107-9b4e-1e517df8d2e5&utm_content=daily
UK MHRA has updated some medical device guidance
Guidance on applying human factors to medical devices – The updates are primarily around this guidance applying to Great Britain, changes in references to legislation and the introduction of the new UKCA mark. https://lnkd.in/eFUrS3C Assistive technology: definitions, examples and safe use – Updates include a new section on the regulations in Northern Ireland. https://lnkd.in/ecvbrYm Medical device safety information produced by the MHRA – The MHRA publishes National Patient Safety Alerts (NatPSA) for medical devices and medicines. When an issue does not meet the criteria for a NatPSA, but still needs to be broadcast, MHRA may issue a different type of communication, such as the MHRA device safety information (which if targeted to you requires a response) and manufacturers’ field safety notices (FSNs). https://lnkd.in/en73gfq
EMA timetable for AA of full MAAs for ATMPs
EMA has published a new timetable for Initial (full) MAA under accelerated assessment for ATMPs.
Here is a link to the document: https://lnkd.in/enq9rEa
(more…)
Last week’s round -up;
08-12 February 2021
UK MHRA is part of the Access Consortium along with the Australia Therapeutic Goods Administration, Health Canada, Health Sciences Authority of Singapore and Swissmedic
The consortium is a medium-sized coalition of regulatory authorities that work together to promote greater regulatory collaboration and alignment of regulatory requirements. The original consortium, formed in 2007 and known as ‘ACSS’, comprised the national regulatory authorities of Australia, Canada, Singapore and Switzerland. In October 2020, the MHRA joined, and the group’s name was changed to ‘Access’. The consortium’s goal is to maximise international co-operation between partners in the consortium, reduce duplication, and increase each agency’s capacity to ensure patients have timely access to high quality, safe and effective therapeutic products. The Access consortium has developed 2 authorisation procedures: the New Active Substance Work Sharing Initiative and the Generic Medicines Work Sharing Initiative. The MHRA commenced work-sharing applications with Access partners from 1 January 2021. MHRA has just added the Expression of Interest (EOI) form for the New Active Substance Work Sharing Initiative to the MHRA website.
This EOI form and further details on the Access consortium are available at the following link: http://bit.ly/3d6sbud
UK MHRA updates Guidelines on UK Orphan Medicinal Products registration
Products with an orphan designation (OD) in the EU can be considered fora Great Britain (GB) orphan MA. A UK-wide orphan MA can only be considered in the absence of an active EU OD. There is no pre-authorisation OD in GB. The MHRA has published a list of authorised orphan medicinal products registered by the UK at the following link: Orphan registered medicinal productsFor GB (England, Scotland and Wales), the MHRA offers incentives in the form of market exclusivity and full or partial refunds for MA fees to encourage the development of medicines in rare diseases. Waiver from Scientific Advice fees will also be available for UK-based SMEs. On grant of a MA with orphan status, the product will benefit from up to 10 years of market exclusivity from similar products in the approved orphan indication. The start of this market exclusivity period will be date of first approval of the product in GB. Market exclusivity periods for Centrally authorised orphan medicine MAs that are converted to UK MAs will continue to apply. Orphan medicines authorised in GB with the results of studies from a paediatric investigation plan (PIP) included in the product information are eligible for an additional 2 years of market exclusivity.
Further details are available at the following link: http://bit.ly/2N1KYMP
EMA product-specific bioequivalence guidance
The EMA has released the following product-specific bioequivalence guidance:
· Draft guidance for Deferasirox Dispersible Tablets, Film-coated Tablets and Granules, which is open for consultation until 31st March 2021.
· New guidance for Levothyroxine Tablets, effective from 1st July 2021.
· Revised guidance for Sorafenib 200 mg Film-coated Tablets.
http://bit.ly/RealCMC-3d6G5fS
Nitrosamines Updates
A new study has reported elevated levels of the potentially carcinogenic nitrosamine NDMA after the ingestion of ranitidine capsules across a range of physiologic conditions, with levels that greatly exceed FDA guidelines under some conditions. These results support the Agency’s request for all ranitidine products to be withdrawn from the market in April 2020. The EMA has released the following updates related to nitrosamine impurities: updated response templates, updated questions and answers guidance document and a new requirement to test medicinal products containing rifampicin for nitrosamines before releasing them to the market.
Further details on the ranitidine study and the EMA nitrosamine updates may be found at the following link: http://bit.ly/RealCMC-3aI3sd8
(more…)
Last week’s round -up;
01-05 February 2021
Pharmeuropa updates
The following draft revised monographs (with tracked changes) have been published in Pharmeuropa. The draft monographs are available for public consultation until 31st March 2021.
· 2.9.2 Disintegration test for solid rectal and vaginal dosage forms
· 2.9.5 Uniformity of mass of single-dose preparations
· Vaginal preparations
The revised monographs can be seen at the following link: http://bit.ly/RealCMC-3rqqXOA
EMA News: EMA announces detail approach on training for the new Clinical Trial Regulation CTIS portal.
In anticipation of CTIS go-live in Dec 2021 to support implementation of the CTR, online training modules are foreseen from 21st Jan to ensure equal access for all. Once launched, CTIS will be immediately available for authorities and clinical trial sponsors, with a 3 year phased transition period from the current Directive 2001/20/EC. EMA plans 3 training streams
1. Creation of Sponsor Master Trainers;
2. micro/SME, academia and other non-commercial sponsors; and
3. Targeting different forms of end-users on role specifics (e.g. admin, preparer, submitter).
Real Regulatory has SME status and will initially train on the 2nd stream. Training and our current involvement in pilot schemes now available will mean we can continue to provide best support to our clients.
Full details can be found here: https://lnkd.in/dUmnKPw
EMA News: EMA has issued a new draft guidance on the Module 3 quality support packages required for MAAs of PRIME Designated Medicines.
PRIME was launched to enhance EMA support to the development of medicines that target an unmet medical need. EMA has just published a draft guidance on scientific elements and regulatory tools to support quality data packages for applications to the scheme. This should serve as a toolbox for building Module 3 quality data packages in the preparation of marketing authorisation applications (MAAs) of designated PRIME medicinal products.
The document in full can be found her: https://lnkd.in/gzTT__J
EMA News: EMA has published detailed accelerated assessment timetables for ATMP full MAAs
The EMA has published a new document which details timetables for full MAA procedures for Advanced therapy medicinal products (ATMPs). Timings extend through to end of 2024, and include assessment of the initial submission (120 day timetable) and timings for assessment of responses to list of questions (30 day timetable after clock-stop).
The document can be found here: https://lnkd.in/enq9rEa
UK MHRA updates Pharmacovigilance System requirements
Following user feedback, the MHRA have edited some of their guidance on Qualified Person responsible for Pharmacovigilance (QPPV) and pharmacovigilance system master files (PSMF) to make it easier to understand and to provide clarification on certain points. The updated guidance can be found here: http://bit.ly/3cAuxkU
UK MHRA publishes the submission dates for the new UK national MAA 150-day procedures
Marketing Authorisation Application submission dates for the new 150-day national and European Commission decision reliance procedures and guidance on how submissions using the EC decision reliance procedure work have been published on the MHRA website, and can be found here: http://bit.ly/3j8GNua
Updates from the EDQM
The EDQM has recently released the ‘New European Pharmacopoeia Technical Guide for the elaboration of monographs on medicinal products containing chemically defined active substances’.
The Directorate has also released the following updates:
• Nitrosamines – Update from the CEP procedure (16-Dec-20)
• European Paediatric Formulary: Phosphate Oral Solution open for public consultation in issue 3 of Pharmeuropa PaedForm (17-Dec-20)
• European Pharmacopoeia Supplement 10.5 now available (04-Jan-21)
• European Pharmacopoeia Commission adopts new general chapter Contaminant pyrrolizidine alkaloids (2.8.26) (05-Jan-21)
• Pharmeuropa 33.1 just released: don’t miss this opportunity to provide your comments (06-Jan-21)
• Brexit: End of mutual recognition of Official Control Authority Batch Release between the EU/EEA and the UK (08-Jan-21)
• Adoption of Fritillariae thunbergii bulbus describing alternative quality control test (15-Jan-21)
• Implementation of the European Pharmacopoeia Supplement 10.5 – Notification for CEP holders (18-Jan-21)
• CEP holders invited to comment on draft monographs published in Pharmeuropa 33.1 (20-Jan-21)
http://bit.ly/RealCMC-3pMWF8o
(more…)
January 2021
Real Regulatory Tips and Insights
The tips we published in January 2021 are collected here, for convenience. Make sure you follow Real Regulatory Ltd and Real CMC for regulatory news, reports and hints.
Type I Variations – Extract(s) from Guideline

eSubmissions Guidance

Editorial Changes for Centralised Product Information

For previous monthly round-ups, you can follow these links:
December 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/december-2020-real-regulatory-tips-and-insights/
November 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/november-2020-real-regulatory-tips-and-insights/
October 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/october-2020-rrl-regulatory-tips-and-insights/












