Services
Real Experts, Real Results
The majority of our work is based around the lifecycle of human medicinal products in Europe, including Advanced Therapy Medicinal Products (ATMPs), New Chemical Entities (NCEs) and known drug substances. We also have in-house expertise to support medical devices and related combination products.
In terms of medicinal products, we can provide EU expert contributors to support products based on small molecules and products of biotechnology. These span from simple synthetic molecules right through to ATMPs based on stem cell or DNA technologies.
Our services cover the full lifecycle of these products from proof of concept right through to product withdrawal.
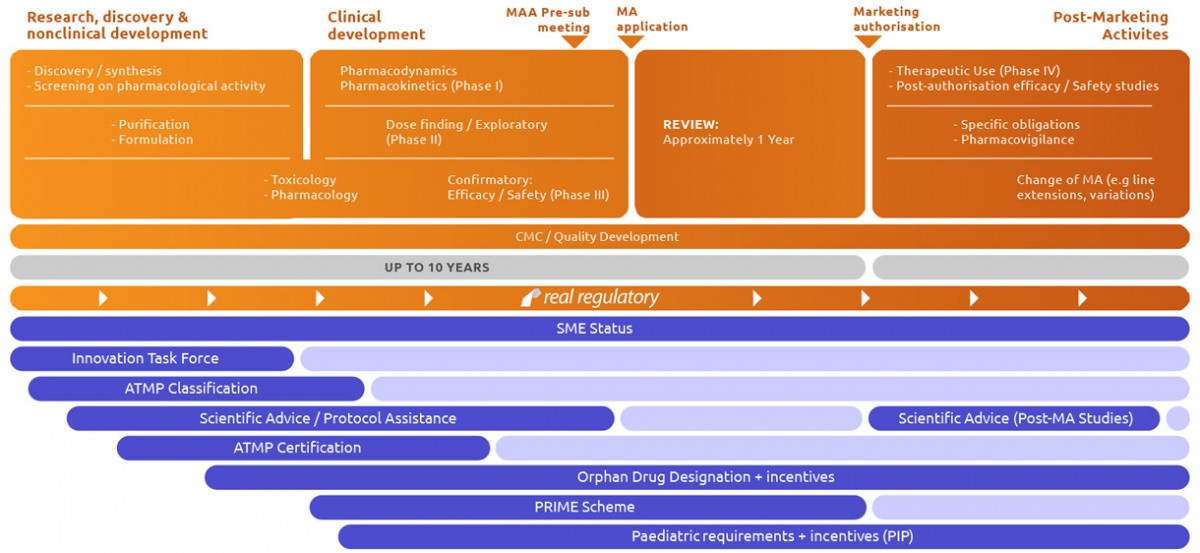
Real Regulatory takes pride in the breadth of our capabilities and our success in working with clients whose needs may be broad ranging. Our extensive panel of external experts provide individual country, language and legislative expertise to complement our internal resources.

