The European Medicines Agency’s Online IRIS Platform: One Year On

The development of drugs to treat rare diseases (orphan drug designation, ODD) is an interesting proposition for drug companies, and designation offers various incentives to sponsors of drugs designated for the diagnosis, prevention or treatment of rare diseases to assist in the development of the product. The European Medicines Agency’s (EMA) new secure online portal, IRIS, aims to make orphan application submissions faster and easier. The use of IRIS became mandatory from 19 September 2018.
Interestingly for an industry that loves to use abbreviations, IRIS is not an acronym, and the EMA explains the origin of the name on its EMA IRIS homepage: “The Iris, or ‘Blue Flag’, is a plant used in healing and medicine since ancient times. In Greek mythology, Iris was a messenger to the Gods who carried the ‘Caduceus’, or staff, now found at the centre of the international symbol for medicine. The IRIS platform facilitates the exchange of regulatory and scientific information between EMA and organisations developing medicinal research products for potential use in the European Union.”
The portal has been designed to allow sponsors to check the status of their applications and receive automatic updates whenever the status changes. IRIS is part of a longer term EMA project that utilises the domains of master data in pharmaceutical regulatory processes (substance, product, organisation, and referential [SPOR]). Sponsors of an orphan drug, or persons acting on their behalf, must have an active EMA account and the EMA account needs to be associated with an industry organisation registered in the EMA’s Organisation Management Service (OMS) before they can use the IRIS platform. To access IRIS, the organisation should request orphan industry “manager” or “contributor” roles via EMA’s Account Management portal.
There are some further administration steps that need to be completed before sponsors can use the IRIS platform and it should be borne in mind that all of these activities require time to set up. It is, therefore, recommended that sponsors start the registration process as soon as possible prior to their targeted orphan designation application submission date.
-
The active substance contained in the orphan application must be registered as “authorised” and “current” in the EU telematics controlled terms (EUTCT) system. The request is made through the EMA service desk. A substance request form (request for inclusion of a new substance in EUTCT), available on the EMA website, should be completed and attached to the service desk request; the EMA also requires a draft version of section A3 (medical plausibility) of the ODD application.
-
A valid research product identifier (RPI), formerly called the “unique product identier (UPI)”, is required for the active substance. This can be acquired via the IRIS platform but only once the active substance is registered in EUTCT, as that information is included in IRIS. The applicant is required to create a submission in IRIS (“Research Product Request Data” is can be selected as the application type).
-
The EMA has issued a useful guidance document, entitled: “IRIS quick guide to registration” EMA/31242/2019 (1), which outlines the required steps prior to accessing the system for ODD and parallel distribution (PD) process related activities (which are to be submitted via IRIS as of February 2019)
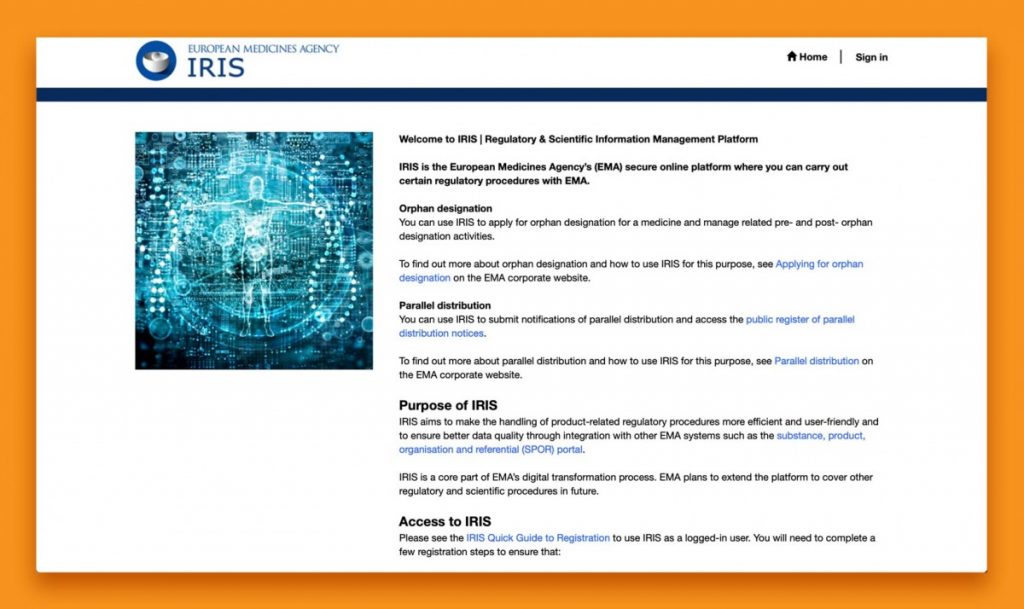
Figure 1 – Screenshot of the IRIS portal

Figure 2 – Screenshot of the SPOR page
Using the IRIS portal
The EMA quick guide EMA/444925/2018 (2) explains the process for logging into and using the IRIS portal (3).
Once a sponsor has completed the initial EMA set-up steps to be able to successfully use the portal, the process of using IRIS is straightforward. Sponsors should still follow the EMA guidance on orphan applications/annual reports and transfers, with the IRIS system providing a means to submit the required documents. For new ODD applications, applicants are still required to provide sections A–E for the scientific part of the application for orphan designation, as well as translations of the active substance name and proposed condition in all EU languages, bibliography, and annexes including proof of establishment of the sponsor in the EEA (see “Guideline on the format and content of applications for designation as orphan medicinal products and on the transfer of designations from one sponsor to another”, ENTR/6283/00 Rev 4) (4). In addition, the portal requires the applicant to provide the administrative information previously included in the separate application form, and the information that is entered generates an application form within IRIS; this form should be attached to the application.
Some post-designation processes have been simplified with the introduction of the portal, for example, the submission of orphan annual reports, where sponsors are no longer required to submit documents but instead just need to complete the relevant fields in the IRIS system (there is an option to upload additional documents, if appropriate). More information regarding this, including the requirements and timelines, may be found in the EMA “Post-orphan medicinal product designation procedures – guidance for sponsors submitting an application via the IRIS online portal” EMA/469917/2018 (5).
When preparing and submitting transfers, the usual appended documents are required (proof of establishment, any translations required depending on decision date for original orphan designation – see checklist for sponsors applying for the transfer of orphan medicinal product designation, EMA/41277/2007 Rev 10) (6). It is worth remembering that both the current and new sponsor should both be registered in OMS. Again, more information regarding this may be found in EMA/469917/2018 (5).
Manager and contributor
The role that an individual has been assigned in the portal determines how much information they can see. Managers are able to see all of the submissions that they have created, as well as anything where they have been assigned as contributor. Contributors will only be able to see the submissions where they have been assigned a contributor role. The “My submissions” page of the portal home page displays “Draft submissions”, “Ongoing submissions” or “Completed submissions”.
Receiving EMA notifcations
When a submission has been made through IRIS, the manager will receive a notification email. If a validation supplementary information (VSI) request is made by the EMA, a notification will be received via email informing that a VSI relating to the ongoing submission is required. List of questions (LoQ) requests will also be received via email notification. Sponsors/applicants should log into the portal and upload any requested documents or make any requested changes. Once all requested changes have been made/information has been uploaded, the user must remember to click on ‘Submit’, otherwise the updated application will not be submitted.
Adoption notifications will also be received via email; sponsors or their contact person should log in to the IRIS portal and download the adoption document. All notification emails are received from info-crm@id.ema.europa.eu.
As with the previous process for applying for or transferring an existing ODD, the European Commission will officially adopt the COMP’s opinion within 30 days of receipt, and a hard copy of the Commission decision is sent to the sponsor or contact person at the address detailed within the application.
Practicalities
In our experience, IRIS is relatively straightforward to use provided that the EMA guidance is closely adhered to and that all of the required initial steps are completed in the correct order. The functionality, and consequently, the guidance, does, however, appear to have been continually evolving since the introduction of IRIS in September 2018, so some of the information in this article may be subject to change and the EMA website should be consulted prior to use.
It should be noted that any orphan designations approved before the launch of the portal were transferred into IRIS by the EMA prior to launch of the system, so moving forward, annual reporting, updates or sponsor transfers for these historical orphan designations should be completed within IRIS. It is, therefore, recommended that sponsors of older orphan designations log into the IRIS system to conrm that their designations have been transferred into the portal correctly. In addition, sponsors may be required to register their active substance and or reference product within the portal (using the steps described above) before they are able to use to the portal to make submissions for their product.
References
- IRIS quick guide to registration, EMA/31242/2019.
- IRIS quick guide to the portal for Orphan Industry Users, EMA/444925/2018.
- IRIS portal. Available at: https://iris.ema.europa.eu/.
- Guideline on the format and content of applications for designation
as orphan medicinal products and on the transfer of designations
from one sponsor to another ENTR/6283/00 Rev 4. - Post-orphan medicinal product designation procedures. Guidance for sponsors
submitting an application via the IRIS online portal, EMA/469917/2018. - Checklist for sponsors applying for the transfer of Orphan Medicinal
Product (OMP) designation, EMA/41277/2007 Rev 10.
The published article can be viewed herein: Regulatory Rapporteur November 2019 p28-29 Spencer and Rackstraw

