The Art of doing Clinical Regulatory well

Introduction
In the EEA, approximately 4,000 clinical trials are authorised each year. This equals approximately 8,000 clinical-trial applications, with each trial involving two Member States on average. Approximately 61% of clinical trials are sponsored by the pharmaceutical industry and 39% by non-commercial sponsors, mainly academia [1].
The clinical trial landscape is becoming more challenging to navigate, with
-
-
-
- Increasingly diverse requirements to fulfil in order to support initial clinical trial regulatory submissions and compliance of the clinical trial post approval
- The upcoming regulatory changes represented by the Clinical Trial Regulations
- ICH E6 requirements of increased sponsor oversight of Clinical Research Organisation (CRO) activities
- Real-time maintenance and oversight of the Trial Master File (TMF)
- Real-time maintenance and oversight of other tracking databases
- Ensuring GCP compliance and inspection readiness.
-
-
These increasing requirements and ever-changing regulatory environment where clinical trial and related data are being ever more scrutinised have created a strain as clinical trial sponsors are trying to achieve their objectives with the same, or reduced, human and financial resources. This creates the need to look carefully at how best a clinical trial sponsor, can utilise their internal team and external service providers to ensure that regulatory best practices are followed and the regulatory and compliance needs of each market are met [2].
A good CRO together with an experienced independent regulatory support team should be able to ease the burden on the sponsor and provide both inspection ready submission material as well as accurate metrics on key performance indicators (KPI’s) such as submission dates, response due dates, etc…
Aside from resolving current issues, the best clinical regulatory approach also focuses on upcoming changes and forward planning. Current regulatory is so much more than expertise in country specific requirements for initial clinical trial submission, but an ability to anticipate issues and identify problem areas.

Clinical trial strategy planning
A successful clinical trial obviously requires a robust clinical strategy. CROs and independent niche experienced regulatory support teams with a global reach can benefit sponsor companies when planning clinical strategies and development activities particularly when key departmental team members from regulatory, clinical, research, development, supply chain and quality are involved in discussions. It is also important to be able to access existing local relationships with: Regulatory Authorities; Clinical Key Opinion Leaders (KOLs) and Ethics Committees.
Globally there is continued learning and improvement to what is considered “state of the art” from a clinical, regulatory and technical perspective, therefore the clinical and regulatory strategy should be regularly reviewed with the CRO and an independent regulatory support team for those countries where the clinical trial is planned to be conducted [2].
Regulatory does not take place in a silo. Changes in any department can have regulatory implications, and vice versa. These need to be taken into consideration and communicated accordingly when discussing strategic options.
At all stages it is important to keep an eye on the bigger picture – e.g. might decisions taken in the clinical trial now affect the eventual marketing authorisation, designations, etc…
Clinical Trial Regulatory submissions: providing best practice ideas for coping effectively with administrative overload in order to meet specified deadlines
There is no magic formula for dealing with the administrative overload that comes with performing clinical trial regulatory submissions in a highly regulated industry sector and in order to meet specified deadlines. Success comes from having a clear and well-communicated strategy, excellent project management and tracking databases, continued communication on progress and good team discipline.
-
For the whole team, internal and external team members – all need to have clearly defined roles and responsibilities to which they are accountable. Teams need to be well supported and have a regular forum where any potential issues can be raised and decisions can be taken to ensure these issues are resolved quickly, so a steering committee with representatives for each functional group/outsourced team is essential [2].

- Each task and document need to have defined timelines and the consequences for the overall project should be communicated and understood if the timeline for each task or document is not achieved. Timelines need to be adhered to, and activities planned accordingly so that the final timeline can be adhered to e.g. planning submissions at least two to three months ahead of the date when a change needs to be implemented in a trial. In some countries this might need to be even six months ahead. One major difficulty in any project is obtaining signatures for sign off so that the next steps in a project can begin. Often the people required are not in the same country, time zone and have other priorities. To compensate for this and keep the momentum and energy high to achieve task closure, it is beneficial to assign for each document a ‘document owner’ who is accountable for obtaining signatures and will dedicate a specific day for signatures [2].
- Previous submissions need to be readily accessible so that they can be easily referred to, thus ensuring that the regulatory history is clear in a particular country.
- The importance of an excellent tracking process with oversight by the regulatory team cannot be over-emphasised. The ever-increasing scrutiny of data, tighter response timelines, sponsor/CRO administrative and resource overload and ever more complex tracking tools – Clinical Trial Management System (CTMS) and Document Management System (DMS) make for a perfect storm. Proper oversight of this tracking process will allow sponsor organisations to monitor key aspects of each of the trial and country submissions such as the initial application and amendment timelines, RFI processes indicators, timer warnings, specific country commitments, approvals, data quality and their overall status. This oversight of the tracking database will also assist in building a font of knowledge of specific country requirements and allow determination of what will be acceptable to authorities in a particular country.
- It is important to keep abreast of changing guidelines (e.g. upcoming Clinical Trial Regulation, Brexit Guidance) and regulatory publications, and identify as soon as possible if changes impact on documentation requirements or other aspects of an ongoing or new trial.
- Additionally, it is necessary to keep in mind short-term pain versus long-term gain. Doing something that seems like the more difficult option in the short term can be better in the long term. e.g. country-specific protocol addenda are easy to do in response to queries received from specific country authorities. However, country-specific protocol addenda can lead to some confusion and greater potential for mistakes when protocols then need to be updated to a new version at a future amendment. Therefore, where it is possible, it is recommended to avoid the creation of country-specific protocol addenda unless it is necessary.
- Recognising and knowing who to turn to for expertise/advice where necessary and ensuring personnel with the most relevant expertise have an opportunity to provide input into the design and documentation associated with the trial will help ensure that the smooth running of the trial.
- An independent experienced regulatory support team will aim to find an acceptable balance between what the sponsor wants to do and what will be acceptable to authorities. The added value in creating an alliance with a niche independent regulatory support team lies in the country specific support provided to the sponsor resulting in consistent and locally compliant CTA submissions and processes to provide evidence of sponsor oversight.

Sponsor oversight of CRO
In recent times, it has become common practice for sponsors of clinical trials to outsource most, if not all, of their activities to Clinical Research Organisations. CROs offer the expertise that sponsors might not have in house, as well as offering supporting roles such as quality control and corrective/preventative actions, activities which would have traditionally been performed by sponsors [3]. While this is of great benefit to sponsors, it does not exempt the sponsor from ultimately being responsible for the quality and integrity of the clinical trial, as per the ICH E6 (R2), “the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor” [4].
Due to the importance of CRO oversight processes today, many sponsors need answers: “Are my CROs doing what I hired them to do?” “Are they adhering to the quality plan?” “Is their performance meeting our expectations?” “What issues should we be escalating? [5]. The challenge therefore for sponsors now consists in oversight of CRO activities. This oversight refers to any measure to control performance, deliverables and efficacy of CROs and sponsors are eager to perform these duties to ensure GCP compliance and so that trial delays can be avoided. Many sponsors now employ the services of several CROs, who may either be working on single or multiple clinical trials at the same time. Maintenance of data coming from multiple sources is difficult to maintain and it is important that sponsors feel the need to be more proactive rather than reactive to issues in clinical trial management. Part of the challenge therefore, is that sponsors are inundated with CRO information in order to keep studies on track. In the past, much of the information was received in notes or spreadsheets, where manual entry itself led to a high error rate. Currently, there are even more complex tracking tools – Clinical Trial Management System (CTMS) and Document Management System (DMS) coming on stream, however even with data available electronically, much of the data needs to be converted from one system (or format) to another and ideally checked to ensure there are no errors or gaps. This requires skills and training on complex databases and processing/checking time —over and above daily tasks for clinical operations teams. Much of that time is spent cleaning up common errors and omissions and checking that agreed KPI’s are met taking valuable sponsor resource away from key clinical competencies [5].
In a benchmark survey conducted across more than 100 clinical leaders from global life sciences companies, 71% of respondents claimed “data transparency and real-time access to quality data” were the top priorities for a successful CRO relationship. In fact, 83% claimed the need to automate CRO oversight, including real-time data aggregation across systems, was “critically significant to success” [5].
The survey assessed gaps and needs, providing insight into how and where sponsors and CROs can improve their collaboration and processes. Key findings included:
-
- CRO oversight is a top priority due to pending ICH E6 updates and increased use of CROs
- Gaps exist in the core CRO oversight execution processes
- Spreadsheets and reactive management are not sufficient for proactive CRO oversight.
A striking observation was that although CRO oversight is a top priority, only 34% of clinical operations leaders indicated that they had successfully achieved their planned CRO goals [5].
The intention and objective of the sponsor to maintain adequate CRO oversight is clearly well-intentioned, but there is a significant gap in sponsors capabilities and a monumental effort is required to close that gap. The addition of an independent regulatory support team allows the sponsor to focus their resources on core clinical competencies, while taking advantage of additional flexible resource throughout the conduct of a clinical trial to bridge the gaps identified in management of CRO oversight activities ensuring compliance and key deliverables are adhered to.
Regulatory activities that are outsourced offer a measure of oversight in CRO activities. Outsourced regulatory activities extend beyond initial submission, amendment submissions and responses with the Ethics Committee or the Competent Authority.
Regulatory services focus on adherence to timelines, both regulatory and internal, with a strong focus on timely completion of relevant submission documentation and accuracy of documentation provided by the CRO. In many cases employing the services of an independent regulatory team will ensure that only completed accurate applications are submitted to the competent authorities, in turn reducing the number of further questions and delays in approval of clinical trials. Regulatory assessment and categorisation of different submission types (substantial, non-substantial), whether grouping of submissions is possible, and anticipation of regulatory authority questions also can assist in meeting deadlines.

Trackers are a valuable tool employed both pre- and post- submission clinical trial applications. Tracking of substantial and non-substantial amendments as well as regulatory commitments are essential to successful management of the trial. Key dates are recorded as well as the status of available documentation. This then simplifies the maintenance of dossiers with the most up to date versions of protocols, investigator’s brochures, investigative medicinal product dossiers, etc. This oversight does not stop at pre-submission activities only. Maintenance of submission records, questions and responses to regulatory authorities and traceability of data changes are equally important in the lifecycle management of clinical trials.
Quality Control (QC) of submissions and review can be performed by an independent regulatory team where necessary, to ensure that specific country submissions are aligned with global strategy. This becomes more important in larger trials which are dealing with diverse local teams and overburdened CROs who may not be fully checking this themselves. These QC checks also ensure that country specific information is included in submission packs. Inspections from national agencies will look for evidence of sponsor oversight in all areas including regulatory submissions. Documentation associated with QC of regulatory submissions which shows the sponsor has reviewed and agreed with the submission can provide such evidence. A QC procedure can ensure that all local regulatory offices provide consistent and high-quality information on the trial to regulatory authorities. In addition, a QC procedure can ensure that the sponsor is aware of all submissions made on their behalf and can file a complete record of all submission documentation. Regulatory sign off and following regulatory procedures for the “green light” process at initial CTA, site initiation and during submission of amendments can also provide evidence of adequate sponsor oversight in this important area.
All the above offer part of a multi-faceted solution to the complex issue of ensuring adequate sponsor oversight of CRO activities and that regulatory best practices are adhered to.
Maintenance of the Trial Master File (TMF)
The Trial master file (TMF) plays a key role in the successful management of a trial by the investigator/institutions and sponsors. The essential documents and data records stored in the TMF enable the operational staff as well as monitors, auditors and inspectors to evaluate compliance with the protocol, the trial’s safe conduct and the quality of the data obtained [6]. Most of the data incoming from the CRO will need to be incorporated into the TMF which needs to be maintained for the duration of the clinical trial and beyond. The trial master file shall consist of essential documents, which enable both the conduct of a clinical trial and the quality of the data produced to be evaluated [7].
Using a CRO eTMF system puts a significant burden on sponsor teams to learn multiple systems and processes. Learning multiple systems from different CROs can become a management nightmare. In addition to the training burden, maintenance of user accounts and validation need to be addressed for each system. The impact of working in multiple systems has a ripple effect throughout the organization and impacts Clinical, Regulatory, Quality Assurance and Data Management teams [8].
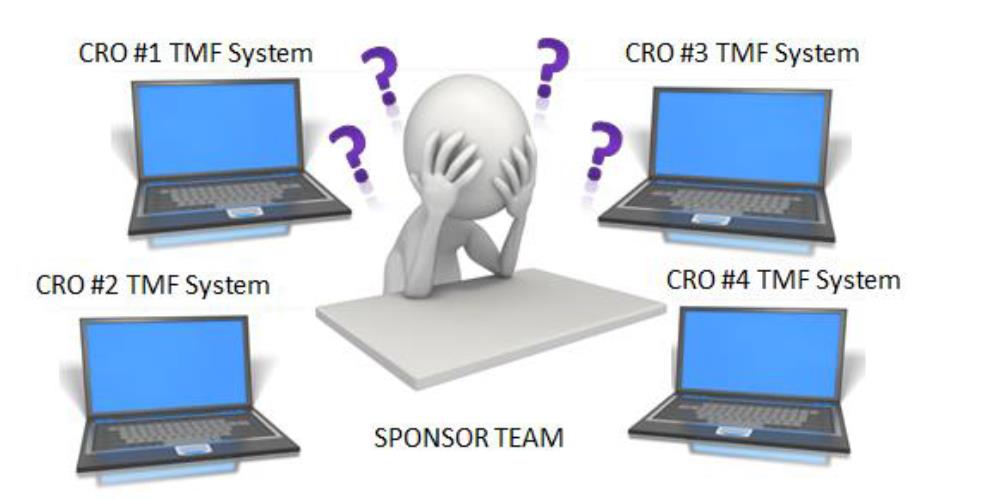
*Picture extracted from [8].
A similar situation arises if the clinical trial sponsor is using their own eTMF system. The CRO is working with multiple sponsors and faces the challenge of becoming familiar with many different sponsor eTMF systems.
The TMF is therefore a critical part of ensuring the compliance of clinical trials, however some of the most common findings in regulatory inspections are related to deficiencies within the TMF and the resources and time required by the sponsor and CRO to create and maintain a compliant TMF are often underestimated.
The added value of creating an alliance with a niche provider of regulatory services (with the time and expertise to familiarise with different systems) and assigning a (regulatory) team to monitor and perform functional quality checks on the TMF ensures constant vigilance and oversight. This approach provides evidence that a proactive approach is being taken, reducing the need for retrospective updates to the TMF which are prone to errors. This in turn would ensure that the administrative burden on the sponsor can therefore be reduced and ensure a well organised and compliant TMF which is GCP compliant and inspection ready.
Conclusion
In conclusion, clinical trial sponsors are challenged everyday with increasing regulatory requirements and an ever-changing clinical trial regulatory environment with tighter timelines, more complex databases, additional demands for clinical data and increased compliance requirements, while still trying to achieve their objectives with the same, or reduced, human and financial resources. These challenges create the need to look carefully at how best a clinical trial sponsor, can utilise their internal team and external service providers to ensure that regulatory best practices are followed and the regulatory and compliance needs of each market are met.
This article discusses the importance of having a robust clinical trial strategy and emphasises the need to keep an eye on the bigger picture so that the future impact of clinical trial regulatory decisions taken now on the eventual marketing authorisation, designations, etc… are considered.
The administrative overload that comes with performing clinical trial regulatory submissions in a highly regulated industry sector with ever more complex databases and tracking tools and in order to meet specified deadlines cannot be underestimated. The best practice ideas introduced above should provide some insights and help sponsors address the key challenges of this administrative burden more quickly and effectively.
It is common practice for sponsors of clinical trials to outsource most, if not all, of their activities to Contract Research Organisations (CRO). While this is of great benefit to sponsors, it does not exempt the sponsor from ultimately being responsible for the quality and integrity of the clinical trial. Sponsor oversight of the CRO remains one of the most challenging and time-consuming aspects of a clinical trial. The addition of an independent regulatory support team allows the sponsor to focus their resources on core clinical competencies, while taking advantage of additional flexible resource throughout the conduct of a clinical trial to bridge the gaps identified in management of CRO oversight activities ensuring regulatory compliance and key regulatory deliverables are adhered to.

This article describes the regulatory activities which can be outsourced to a regulatory service provider and discusses the way in which these outsourced regulatory activities offer a measure of oversight in CRO activities. Regulatory services focus on adherence to timelines, both regulatory and internal, with a strong focus on timely completion of relevant submission documentation and accuracy of documentation provided by the CRO. Additionally, the employment of trackers and the use of a robust quality control procedure which provides evidence of sponsor oversight of content and timing of submissions were discussed.
Finally, the challenges associated with maintaining a well organised and compliant TMF which is GCP compliant and inspection ready were described. The added value of assigning a regulatory team to monitor and perform functional quality checks on the regulatory portion of the TMF ensures a constant vigilance and oversight. This approach provides evidence that a proactive approach is being taken which will facilitate compliance and inspection readiness.
All the above insights offer part of a multi-faceted solution to address some of the complex issues associated with running global clinical trials in an increasingly challenging environment. Maximum preparedness and forward planning are the keys to ensuring that the art (or perhaps more aptly the science!) of doing clinical regulatory well is achieved.
References:
[1] EMA website https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials-human-medicines
[2]Clinical Trials Arena News August 2015: Embedding the valuable regulatory knowledge from your service providers for your device trial: https://www.clinicaltrialsarena.com/news/embedding-the-valuable-regulatory-knowledge-from-your-service-providers-in-order-to-meet-the-demands-of-the-country-you-choose-to-work-in-for-your-device-trial-4644551-2/ [3] Hennig M et al. Current practice and perspectives in CRO oversight based on a survey performed among members of the German Association of Research-Based Pharmaceutical Companies (vfa) GMS German Medical Science 2017, Vol. 15, ISSN 1612 3174 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5278541/ [4] EMA/CHMP/ICH/135/1995: Guideline for good clinical practice E6(R2) https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf [5] Peacock J. Applied Clinical Trials: Achieving CRO Oversight: Strategy at Comprehend Systems, Dec 2016: http://www.appliedclinicaltrialsonline.com/achieving-cro-oversight [6] EMA/INS/GCP/856758/2018: Guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-content-management-archiving-clinical-trial-master-file-paper/electronic_en.pdf [7] European Directive 2005/28/EC https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/dir_2005_28/dir_2005_28_en.pdf [8] Redding K. Phlexglobal White Paper: 2014 Phlexglobal Ltd. Points to Consider When Developing a TMF (Trial Master File) Strategy http://www.samedanltd.com/uploads/pdf/white_paper/dc3c7e1434ba5bd3ffe62cee1bdefb9a.pdf

