Ireland’s Ambitions and Innovation for Drug and Research Development

Introduction
Ireland’s reputation as a centre of excellence for manufacturing activities, in particular in drug development, is well known. In 2015, Ireland was home to nine of the top ten global pharmaceutical companies and 17 of the top 25 global medical device companies (1). In 2018, 18 of the world’s top 20 pharmaceutical companies and 15 of the top 25 global medical device companies were located in Ireland (2).
Manufacturing activities and research and development (R&D) are critical elements of health innovation and economic growth in Ireland. However, although there has been significant investment in manufacturing activities, R&D investment has lagged behind, particularly in clinical trials. Recognising its importance, Ireland has targeted investment in this arena and is striving to become a centre of excellence for R&D within the health and medical sector. A strategy for realisation of this objective is detailed in a 2015 government document: “Innovation 2020 Excellence Talent Impact”, (1) which is driven by the Department of Business, Enterprise and Innovation. The document details Ireland’s five-year plan for R&D, science and technology, setting out a roadmap to deliver excellence, talent and impact in R&D (1).The government identified and is engaging with all stakeholders to deliver on R&D in health research. The third progress report following the 2015 document, published in July 2018, (3) notes significant progress on implementation of the plan.
Ireland is listed among the top 20 countries in global rankings for the quality of scientific research since 2009, with its ranking in citations moving up to 11th place in 2018 (1, 4). Improving and maintaining excellence in R&D through prioritising public and private investment in RDI is a key objective of Innovation 2020 with a vision for Ireland as an internationally competitive research system that acts as a magnet and catalyst for talent and industry (1). The Prime Minister of Ireland at the time the Innovation 2020 document (1) was first published wanted Ireland “to be the best small country in the world in which to do business”. The Irish government recognised that Ireland cannot be a leader in all areas of enterprise, research and innovation and therefore targeted investment in 14 priority areas across six broad enterprise themes. Two of these six themes are central to strengthening drug development in Ireland, namely manufacturing and materials, and health and medical. Research Prioritisation (RP) also identified the need to support relevant enabling technologies to underpin the priority areas including basic biomedical science, nanotechnology and advanced materials (1).
The government has engaged with all relevant stakeholders to deliver on its objectives and understands that collaboration between all stakeholders is required to drive RDI. This article discusses some of the mechanisms by which progress is being achieved within the innovative drug development landscape as Ireland strives to meet its objectives on RDI.
Clinical trials in drug development
Cancer research trials organisations such as Cancer Trials Ireland (formerly ICORG) support clinical trials of novel medical therapies such as the one described in the Oncotype DX test case below. This type of investment in the Irish clinical trial arena will help Ireland attain a centre of excellence status for R&D in the health and medical sectors.
The Oncotype DX diagnostic test. Some women with early-stage breast cancers have a reduced risk of cancer recurrence following chemotherapy, while some do not. Inability to distinguish these sub-groups upfront has been a clinical problem. A new diagnostic test (Oncotype DX), (1) which analyses the expression pattern of 21 genes in breast tumours, promised to address this problem by guiding the clinical decision as to whether or not to give chemotherapy. The clinical trial investigating the validity of this diagnostic test was sponsored by the National Cancer Institute (US) using public funding. Irish participation in this international trial was funded by the Health Research Board (HRB) through the cancer clinical trials network (formerly) ICORG. Ireland was the largest recruitment
centre outside the US, with approximately 700 women participating.
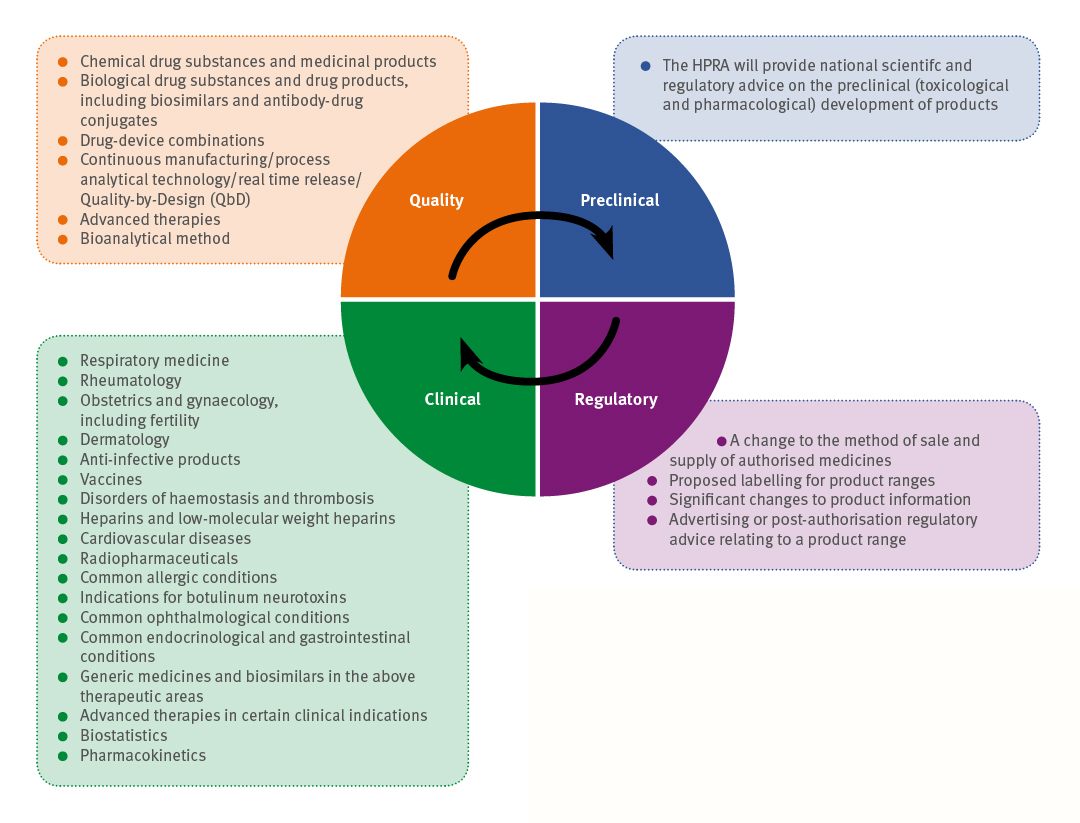
Figure 1: The types of scientific advice given within the four categories of quality, preclinical, clinical and regulatory
Over the four-year clinical trial, there was an estimated cost saving to the Irish health system of €5m. Based on these positive outcomes, Ireland became the first country in the world to reimburse the use of Oncotype DX as a diagnostic for routine cases (1).
Ireland is increasingly becoming the locale of choice for multinational companies to test cutting-edge technologies and to conduct trials of new products, often in conjunction with public bodies. Ireland’s size – “small enough to trial, large enough to prove” – its unique geographic and environmental characteristics, and the interconnected public sector makes Ireland ideal for testing and validating technologies in real-world conditions, before bringing them to a wider market (1).
From a regulatory perspective, the Health Product Regulatory Authority (HPRA) (competent authority – CA) is responsible for the assessment of clinical trials involving medicinal products in Ireland. The types of trials assessed range from first-in-man studies for new compounds to studies with products which already have marketing authorisations (6). In addition to CA approval, a positive opinion is required from one of the 12 Irish research ethics committees (RECS) before a clinical trial can be conducted in Ireland.
The new Clinical Trial Regulation
The Irish government also sees promotion of standards and regulations as a source of competitive advantage for Ireland. Regulatory changes in clinical trials will see the implementation of the new clinical trial regulations which will help improve outcomes of trials in terms of getting safe, effective medicines to patients in the shortest time possible.
The new Clinical Trial Regulation (CTR) will ensure a greater level of harmonisation of the rules for conducting clinical trials throughout the EU. The regulation introduces an authorisation procedure based on a single submission via an EU portal with an assessment procedure leading to a single decision. The changes will ultimately result in harmonisation across all EU trials, supporting sponsors in achieving approvals in multiple EU regions with greater efficiency (6). The new Regulation also addresses ethics committee (EC) involvement in the assessment process, allowing sponsors to get the approval for the EC in parallel or subsequent (within two years) to the CA approval (6).
The HPRA has been working on implementing the new regulation by setting up a National Pilot Project phase. This is a voluntary project which will give the sponsor an opportunity to submit to the HPRA and EC in order to obtain a single national decision for the clinical trial (if feasible). The sponsor will be required to submit the documentation under the new regulation format with Part I and Part II. This pilot phase will be supported by the new planned National Ethics Committee (5).
Clinical trials – infrastructure
National Research Ethics Committee. In conjunction with Ireland’s Innovation 2020 strategy and, in a significant step forward for Health Research, the Irish government has approved proposals by the Minister for Health to prepare a Bill to support a mixed model national REC system (7).
This would establish a national ethics committee infrastructure that will work alongside and support the existing local or institutional RECs.
The Health Research Board (HRB), a state agency under the Department of Health whose main business area is funding research proposals and managing grants, has welcomed the Bill stating that “the establishment of a single, cohesive national REC structure in Ireland will help grow health research and clinical trial activity that will benefit people’s health and patient care, as well as underpinning health innovation and economic growth in Ireland” (8). The proposed new national REC system will address concerns raised by stakeholders over the past decade about the existing system in Ireland for clinical trials and health research generally. This approach will maximise synergies and value-for-money outcomes and make Ireland a more attractive international location for all health research (7).
The Bill is regarded as a priority and the Department of Health will work closely with stakeholders, including the HRB and the HPRA to have it published by the end 2019.7 In the context of the preparation of the General Scheme for the Bill, the Department will engage with a broad range of stakeholders, including other government departments, agencies, institutional RECs, researchers, industry and patients to seek their views on specific matters.
European Clinical Research Infrastructure Network. Another significant development welcomed by the HRB and other stakeholders is that Ireland officially joined the European Clinical Research Infrastructure Network (ECRIN) as a Member Country in December 2018 (9). ECRIN’s Irish national scientific partner is the HRB and Clinical Research Coordination Ireland (CRCI), an independent, integrated national clinical research network that provides centralised support for the conduct of multicentre clinical trials (commercial and academic) across Ireland.
The HRB issued a press release in which it stated that ECRIN membership will increase access for Irish patients to multinational clinical trials and make it easier for Irish researchers to extend their trials internationally and help improve patient safety and quality of care (10). Ireland’s membership brings ECRIN’s number of member countries to nine (Czech Republic, France, Germany, Hungary, Ireland, Italy, Norway, Portugal and Spain), with two observer countries (Switzerland and Slovakia) (9).
Scientific advice. The HPRA is a key stakeholder in health research and in 2016 it established a new scientific advice scheme on a pilot basis. Due to the scheme’s success, the pilot study was extended into 2017 based on several national scientific advice meetings. It is now fully operational in the HPRA, providing national scientific and regulatory advice to commercial and non-commercial entities. The overarching aim is to assist applicants in the development of new or existing human medicinal products by taking into account the current knowledge of a given condition, targeted patient population, existing treatment modalities and specificities of the product being developed (11). The advice may assist applicants in the confirmation of guidelines, provide information where guidelines do not exist on regulatory aspects or provide assistance to non-commercial bodies such as academics intending to submit a clinical trial application. Since its establishment in 2016 the scientific procedure has grown on a yearly basis and become a national success, and is a great addition to the growing R&D industry in Ireland. In 2016, during the pilot scheme, it held six scientific advice meetings, while there were 13 in 2017 and a further 13 in 2018.
The advice provided can be related to scientific development, clinical trial application(s), regulatory advice or a combination.
Since the establishment of the HPRA’s national scientific advice procedure it has been continuously expanding to include additional therapeutic areas. The type of scientific advice provided has been broken down into four categories: quality, preclinical advice, clinical advice and regulatory advice (12) (see Figure 1) (11).
The HPRA’s clinical and scientific expertise ensure health products are as safe as possible and do what they are intended to do. General regulatory advice is also provided to applicants and marketing authorisation holders outside the scientific advice procedures, e.g., during assessment, via emails and face-to-face meetings.
Innovation Office. The Innovation Office provides advice at an earlier stage of product development along with scientific advice provided at an EU level through the European Medicine Agency’s Scientific Advice Working Group (SAWG). The Innovation Office provides regulatory support from an early stage in the development of innovative products. Supporting innovation is a key strategic goal of the HPRA due to the high density of innovative companies across the life sciences sector in Ireland and the presence of extensive research and development. Due to strong links between academia, industry and regulators, Ireland’s innovation sector is thriving (13).
Conclusion
Ireland has taken major steps forward in positioning itself as a centre of excellence for research in drug development over the past five years and is continuing to strive to be a centre of excellence by 2020. It has achieved progress in this arena via investment in knowledge, skills, expertise and infrastructure in health research and development. Significant progress has been made in providing the environment and regulatory landscape for conducting clinical trials in Ireland, in particular the introduction of the Bill to establish a single, cohesive national REC structure. The HPRA has also contributed greatly to the regulatory landscape in both Ireland and the EU and has earned an excellent reputation in regulatory compliance.
References
(1) Interdepartmental Committee on Science, Technology and Innovation. Innovation 2020 – Excellence, talent, impact. December 2015. Available at: https://dbei.gov.ie/en/Publications/Publication-files/Innovation-2020.pdf
(2) Enterprise Ireland Life Sciences brochure. Available at: https://www.enterpriseireland.com/en/Start-a-Business-in-Ireland/Startups-from-Outside-Ireland/Key-Sectors-and-Companies-in-Ireland/Medical-Devices-sector-profile.html
(3) Innovation 2020 – Excellence talent impact 3rd progress report. Available at: https://dbei.gov.ie/en/Publications/Innovation-2020-Progress-Reports.html
(4) IDA Publication. Facts about Ireland 2018. Available at: www.idaireland.com/newsroom/Publications/Facts_About_Ireland_2018.
(5) List of RECs in Ireland recognised under the 2004 Regulations. Available at: https://health.gov.ie/wp-content/uploads/2016/07/European-Communities-Clinical-Trials-on-Medicinal-Products-for-Human-Use-Regulations-2004.pdf.
(6) Health Products Regulatory Authority. Clinical trials. Available at: www.hpra.ie/homepage/medicines/regulatory-information/clinical-trials.
(7) Department of Health. Minister for Health secures Government approval to create national research ethics committee. Press release 7 February 2019. Available at: https://health.gov.ie/blog/press-release/minister-for-healthsecures-government-approval-to-create-national-research-ethics-committee/.
(8) New Bill for national research ethics committees. Health Research Board News 7 February 2019. Available at: www.hrb.ie/news/latest-news/newsstory/article/new-bill-for-national-research-ethics-committees/.
(9) ECRIN (European Clinical Research Infrastructure Network). Available at: www.ecrin.org/news/ireland-joins-ecrin-member-country.
(10)Ireland to join European Clinical Research Infrastructure Network. Health Research Board Press Release. Available at: https://www.hrb.ie/news/press-releases/single-press-release/article/irelandto-join-european-clinical-research-infrastructure-network/.
(11) Health Products Regulatory Authority. HPRA guide for national and scientific and regulatory advice. Available at: www.hpra.ie/docs/default-sourcepublications-forms/guidance-documents/ADV-G0017-guide-for-nationalscientific-and-regulatory-advice-v4.pdf?Status=Master&sfvrsn=23.
(12) Health Products Regulatory Authority. HPRA newsletter Issue No 62. Available at: www.hpra.ie/docs/default-source/publications-forms/newsletters/hpra-medicinal-products-issue-number-62-final-for-web.pdf?sfvrsn=9.
(13) HPRA Innovation Office. Available at: www.hpra.ie/docs/default-source/publications-forms/information-leaflets/hpra-innovation-dl-(web).pdf?sfvrsn=4.
The published article can be viewed herein: Regulatory Rapporteur September 2019 p21-23

