ICH Q12: A European Perspective

Introduction
The much-anticipated ICH guideline Q12 has been released in its final version. The guideline has been in development since 2014 and deals with post-approval changes to chemistry, manufacturing and controls (CMC). One of the guideline’s underlying aims is to globally harmonize such CMC change management, and, as covered back in 2016, industry stakeholders had high expectations that it would significantly reduce regulatory burden, related costs and approval timelines.
However, it is up to each regulatory region to choose how to implement Q12, and in Europe this has come with a disappointing catch. Due to the current EU legal framework, the guideline cannot be fully implemented, reducing the impact that it can have on currently established practices. Herein, I outline the guideline’s main principles and highlight what can and cannot be implemented within the EU at this time.

Overview of ICH Q12
In its final form, Q12 further intertwines regulatory affairs and quality assurance functions in relation to CMC changes. The guideline concurrently emphasizes additional reliance on a company’s Pharmaceutical Quality System, whilst reducing regulatory oversight. This move is only possible using a number of complementary regulatory tools and enablers, some of which Marketing Authorisation Holders (MAHs) will already have in place, whereas others are newly introduced.
The kick-off point is product and process knowledge, which allows establishment of an appropriate control strategy, elements of which are considered to be Established Conditions (ECs). Q12 defines ECs as legally binding information considered necessary to assure product quality. Conversely, other information is considered to be supportive.
All CMC changes to an approved product are managed through an MAH’s Pharmaceutical Quality System (PQS), and changes to ECs must also be reported to the relevant regulatory authority. Related reporting requirements are based on risk categorization with respect to potential effects on product quality, safety, and efficacy.
Prior agreement between the MAH and the regulatory authority in terms of the requirements and studies needed to implement a change post-approval are facilitated through Post-approval Change Management Protocols (PACMPs). A summary of expected lifecycle management, called the Product Lifecycle Management Document (PLCM), further strengthens this transparency within the Marketing Authorisation
Application (MAA). Regulatory assessment and inspection are expected to act complementarily to enable guideline implementation.
Q12’s scope covers both chemical and biological drug substances and products, as well as drug-device combination products that meet the definition of a pharmaceutical or biological product. No distinction has been made between originator or generic products, and the guideline also indicates that its principles can be used both for new applications and already marketed products.
A more detailed look at ICH Q12’s main pillars and EU implementation
Categorisation of Post-approval CMC Changes
ICH Q12 advocates for a risk-based categorization in relation to regulatory communication requirements. The reasoning behind this is that CMC-related changes vary widely when it comes to their potential effects on product quality, safety and efficacy.
Using such a framework, prior approval is needed for changes that are deemed sufficiently risky that they require detailed information to be submitted to the regulatory authority for review. Changes that require less supporting information can be communicated to the authority as a formal notification, without the need for prior approval. (Such reporting may need to take place before or after implementation, depending on regional requirements.) Changes that are not required to be reported to regulators should be managed through the company’s PQS.
The EMA has pointed out that similar risk-based principles already guide reporting requirements within the EU, and that these will remain as per the current EU Variation Guidelines. However, one of the main differences between ICH Q12 and the EMA’s position is with regards to Established Conditions.
Established Conditions
Within ICH Q12, ECs form the basis for whether a post-approval CMC change needs to be reported to a regulatory authority. Changes to high risk ECs must be reported for approval, low-to-moderate risk ECs can be notified, whereas non-ECs do not need to be reported.
Their identification is based on knowledge gained throughout the product lifecycle. This includes pharmaceutical development and characterization of the drug product and substance. MAHs should clearly identify and justify both ECs and supporting information, together with their associated reporting categories, within the appropriate CTD modules.

Q12 describes various scientific risk-based approaches that enable EC identification for both manufacturing processes and analytical procedures. It also includes a handy appendix that highlights the various CTD modules in which ECs are generally found, as well as more comprehensive annexes to further enable identification. The guideline emphasizes that its use should not lead to less detailed descriptions within analytical or process sections.
Although the term ‘Established Conditions’ does not exist in the EU legal variation framework, the EMA has pointed out that these mirror information and quality characteristics that are currently subject to a variation under the current EU Variations Regulation and associated EU Variation Guidelines. The authority has emphasized that definition of ECs and reporting categories must follow the requirements laid down therein. It has also underlined that the additional scientific risk-based approaches to defining ECs and associated reporting categories that are outlined within Q12 are considered incompatible with the EU’s current legal framework and will not be accepted in the region.
Post-approval Change Management Protocol
The Post-approval Change Management Protocol (PACMP) serves as a pre-agreement between the MAH and regulatory authority on the requirements and studies needed to implement a change post-approval. PACMPs can be used within the EU. In fact, this capability has been in place for a number of years and one of the expectations of the ICH Steering Committee is that Q12 will increase their use within the EU, facilitating continual improvement of the product and process, and reducing the need for Type II variation submissions.
Q12 outlines the typical elements expected within a PACMP, which can be located in CTD Module 3.2.R. An MAH is expected to demonstrate product and process understanding, and include the attributes listed within Table 1.
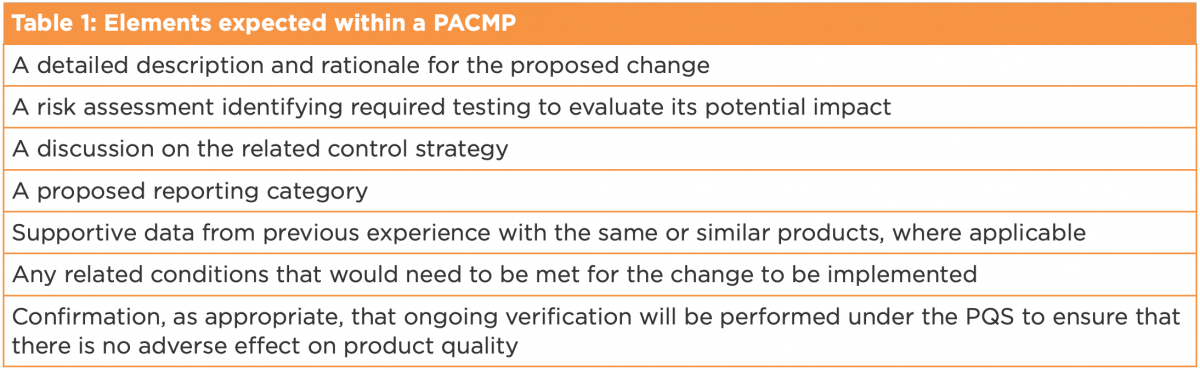
A PACMP can be submitted with the original MAA or as a subsequent standalone submission. Either way, it needs to be approved by the regulatory authority before its execution. There are different types of PACMPs described in ICH Q12: those associated with a single product, and broader protocols across multiple products or multiple sites. Q12’s annexes include comprehensive examples for each.
Once approved, execution of the protocol by the MAH would then follow, after which related results are submitted to the regulatory authority according to the previously agreed-upon categorization. Notably, Q12 highlights that PACMPs cannot be used for any CMC change that would require supportive efficacy, safety or human pharmacokinetic/pharmacodynamic data.
Product Lifecycle Management Document
The Product Lifecycle Management Document (PLCM) brings together the ECs of a product, reporting categories for changes to these ECs, PACMPs, and post-approval CMC commitments. The underlying aim is to facilitate prospective and ongoing lifecycle management and improve related transparency between MAHs and regulators.
Q12 specifies that this document should be included in the original MAA, located similarly within CTD Module 3.2.R, and updated in post-approval CMC submissions. It should also be made available to inspectors, so that they’ll be aware of the current status of PLCM elements, with their related inspection conclusions being used to support ongoing regulatory oversight. Q12’s annexes include a detailed example of this document as well.
However, benefits of the PLCM will not be accessible in the EU as the EMA has made it clear that this document will not be accepted in the region, due to incompatibility with the current legal framework.

In conclusion
Although the underlying aim of Q12 is to globally harmonize post-approval CMC change management, regional discrepancies will remain as its implementation depends on related regulatory frameworks. Within Europe, the guideline’s potential benefits cannot be accessed in full, though it should still bring added transparency and proactivity to the management of post-approval CMC changes. It is also worth noting that the EMA has indicated that currently excluded elements will be considered during the next review of the EU’s current legal framework.
On the other side of the coin, maximization of potential benefits from Q12 also depends on buy in from pharmaceutical companies. Robust Pharmaceutical Quality Systems are required, including effective change management systems and clear communication pathways between the MAH and all suppliers and contract manufacturers within a manufacturing chain.
References
EMA/CHMP/CVMP/QWP/586330/2010. Questions and answers on post approval change management protocols. Accessible from: https://www.ema.europa.eu/en/questions-answers-post-approval-change-management-protocols
EMA/CHMP/ICH/804273/2017. ICH guideline Q12 on technical and regulatory considerations for pharmaceutical product lifecycle management.
Accessible from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q12-technical-regulatory-considerations-pharmaceutical-product-lifecycle-management_en.pdf
EMA/CHMP/ICH/78332/2020. Note on EU implementation of ICH Q12 (guideline on technical and regulatory considerations for pharmaceutical product lifecycle management). Accessible from: https://www.ema.europa.eu/en/documents/other/note-eu-implementation-ich-q12-guideline-technical-regulatory-considerations-pharmaceutical-product_en.pdf
European Commission Guideline 2013/C 223/01. Guidelines on the details of the various categories of variations, on the operation of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 of 24 November 2008 concerning the examination of variations to the terms of marketing authorisations for medicinal products for human use and veterinary medicinal products and on the documentation to be submitted pursuant to those procedures. Accessible from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52013XC0802(04)
Fiorini Cohen T. (2016) ICH Q12 – Where is it at and where do we want it to go? Accessible from: www.linkedin.com/pulse/ich-q12-where-do-we-want-go-tessa-fiorini-cohen
ICH (2014). Final Business Plan Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management. Accessible from: https://database.ich.org/sites/default/files/Q12%20Buisness%20Plan.pdf

