Good Clinical Practice for Clinical Trials

Overview
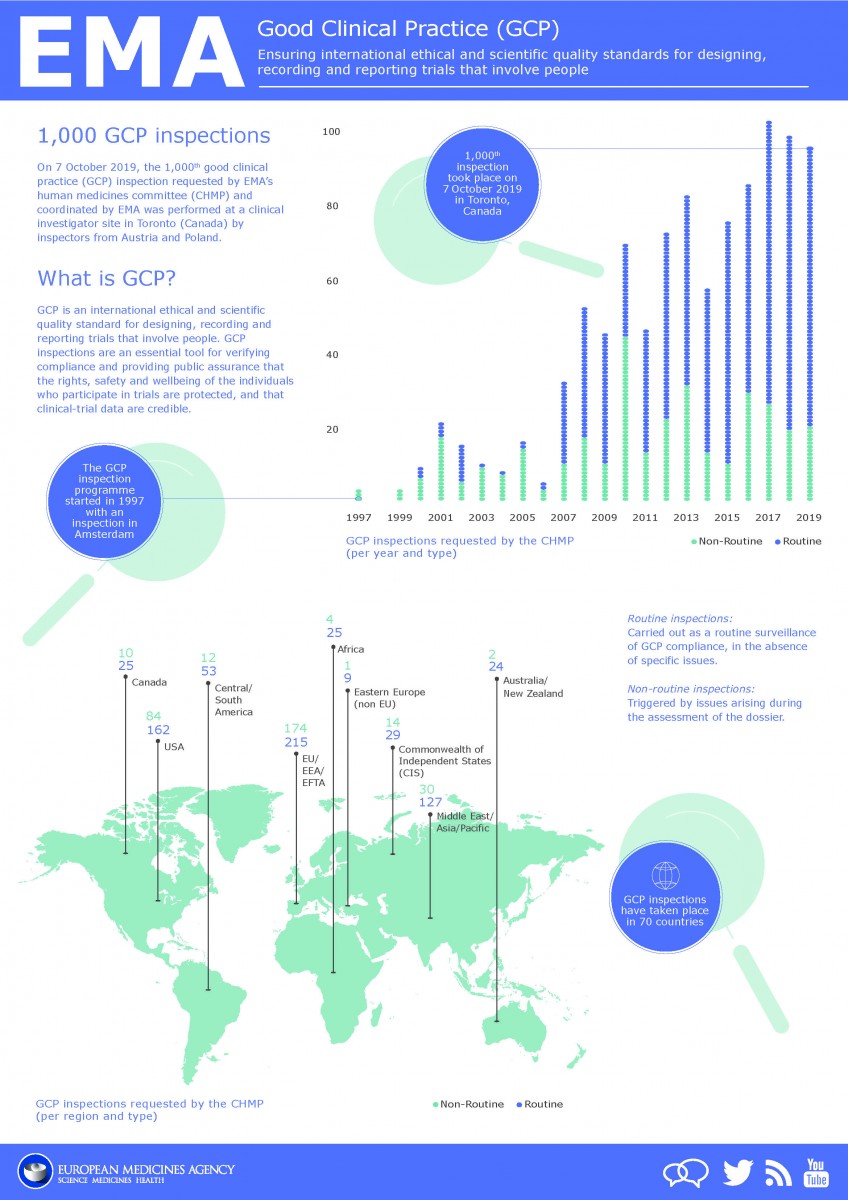
Good clinical practice (GCP) is an international ethical and scientific standard for designing, conducting, recording and reporting trials that involve the participation of human subjects. Clinical trials are conducted worldwide by pharmaceutical companies, contract research organisations, universities and charities with GCP compliance assessed via GCP Inspections. EMA performed the 1st GCP inspection in 1997, the 1,000th GCP inspection requested by EMA’s CHMP and coordinated by EMA was performed on 7th Oct 2019 as shown on infographic below. GCP Inspections can be routine which are carried out as a routine surveillance of GCP compliance, in the absence of specific issues or non-routine which are triggered by issues arising during the assessment of the dossier.
This article informs persons performing clinical trials how MHRA GCP inspections are conducted and more importantly of key findings from such inspections conducted from April 2016-March 2017. MHRA guidance document ‘GCP for clinical trials’ addresses how to show the MHRA that you meet GCP standards and what to expect from an inspection. MHRA ‘GCP Inspection Metrics 2016-2017’ reports on the types of findings resulting from 99 GCP Inspections (Commercial, Non-Commercial, CRO’s, Investigator sites, Phase 1 units) categorised as critical, major and other.
MHRA GCP Inspections
MHRA acts as the regulatory body and as such perform regular inspections to assess compliance to GCP as follows:
-
- asks trial sites to notify them of serious breaches
- carries out inspections of trial sites where serious breaches are reported
- carries out inspections of trial sites that sponsor clinical trials, mostly based on a risk assessment score
- carries out inspections of sites when companies apply for marketing authorisations.
Serious breaches may trigger an inspection however the majority of MHRA GCP inspections at trial sites are carried out under the risk-based compliance programme which are addressed below.
Inspections under the risk-based compliance programme
System based or trial specific.
GCP systems inspections are undertaken to determine the systems used by the sponsor in conducting clinical trials. The MHRA uses information from previous inspections as well as any
- organisational changes to determine the need for risk-based inspections. Several of the Sponsor’s ongoing clinical trials or even several study sites within a clinical trial can be assessed during these inspections.
- trial specific inspections investigate clinical trials that have already been completed and reported.
Currently phase I units that are part of the Phase I accreditation scheme are not part of the risk-based programme, but they are inspected every 2 years.
Since 2016, routine GCP compliance reports are no longer required to be submitted to the MHRA. Should the need arise, the MHRA is entitled to ask the Sponsor to submit such a compliance report.
In a no-deal Brexit, the MHRA’s GxP risk-based inspection programmes will remain unchanged.
Inspection process – Expectations
Prior to the inspection, the Sponsor will be notified of the intent to inspect the organisation. Certain documentation will be requested from the Sponsor, including a list of ongoing clinical studies, organizational charts, standard operating procedures lists and overview of the facilities. The MHRA provides online supports for inspection preparation. The GCP inspection dossier template and GCP inspection clinical trial spreadsheet are available to download on the MHRA website.
The trial master file must be available during the inspection. The TMF is the basis for the inspection and any documents in it must be made available for inspection, regardless of whether these documents are subcontracted to a CRO. Anticipated inability to provide the trial master file should be communicated to the investigator ahead of the inspection.
Additional documentation and key personnel may be requested during the inspection, which must be made available. The inspector may also visit some of the sites involved in the clinical trial such as pharmacies and laboratories.
Inspection Outcome
A verbal summary will be provided at the end of the inspection. This presents an opportunity for any misunderstandings to be addressed ahead of the final inspection report, which will be provided by email at a later date. Findings will be categorized as either minor, major or critical. An email response will be required to address any findings and to give an overview of any corrective or preventative actions resulting from the inspection.
Critical findings will be further reported to the GCP IAG (Inspection action group), and additional actions may result from their review of the findings. These actions will depend on the critical finding and the impact to human safety and data integrity. These actions can include quarterly reporting, early re-inspections, referral to relevant authorities, suspension of clinical trial applications and infringement notices or prosecution.
GCP Compliance Tools
To help relevant parties comply with GCP regulations, the MHRA has put together several tools to help achieve compliance as detailed below.
The GCP compliance forum allows researchers to discuss GCP regulations.
GCP Stakeholder Engagement Meetings (StEM) are now held annually, providing a forum of discussion between GCP Inspectorate and key stakeholders. The GCP StEM focuses on the conduct of clinical trials of investigational medicinal drugs.
The annual GCP Laboratories Stakeholder meeting also provides a forum of discussion between the MHRA GCP inspectorate and stakeholders on issues pertaining to the analysis of samples from clinical studies.
GCP Inspection Metrics Report – Inspection Findings
The Inspection Metrics Report covers inspection findings for 99 GCP audits of commercial sponsors, non-commercial sponsors, CRO’s, Investigator sites and Phase I units over a 1-year period (April 2016-March 2017). There were 14 critical findings in total given for the following GCP breaches:
-
- Pharmacovigilance
- Record Keeping /Essential Documents-TMF
- Clinical Trial Authorisation
- Data Management – Data Integrity and monitoring
- CSV- Computer System Validation
- Monitoring
- Subject Eligibility
The findings are detailed in the report and the highest number of findings relate to pharmacovigilance, TMF and monitoring which are summarised below.
Pharmacovigilance GCP breaches related to the use of unapproved updated Regulatory Summary Information (RSI), failure to use approved RSI for expectedness assessments and therefore the risk of inaccurate reporting of SUSARs and the potential for SUSARs to go unreported and inaccurate information being submitted in DSURs due to failing pharmacovigilance processes. There was a lack of control of the submission of the RSI to the REC and the MHRA, as evidence was seen that there was no consistency between the RSIs that were submitted to them. GCP breaches relating to Record Keeping/Essential Documents-TMF included missing essential documents from TMF, TMF not clearly defined in terms of ancillary systems and documents located within them, difficulty in ready availability and accessing of documents and poor oversight/quality control of TMF maintenance.
There was a critical finding for monitoring by the sponsor whereby the sponsor failed to identify the recruitment of patients who were ineligible for participation within the range of trials reviewed.
The vast majority of these findings resulted from shortcomings in terms of sponsor oversight of CRO activities. Sponsors need to ensure their processes in terms of oversight of CRO are robust, effective and that implementation of the processes is continuously monitored.
The MHRA Inspection Metrics Report is a useful document for stakeholders to use as a tool for internal auditing of clinical trials they are conducting and for establishing processes and procedures for conducting future clinical trials.

